Author: Nicole Brown
January 10 – 10 min read
What’s new, what’s next, and what it means for our business.
Published monthly, the OBX Innovation Briefing is presented by the OBX team as a way of highlighting the latest developments and innovations within the global cannabinoid industry. Each curated volume features insights and commentary on industry and category trends, regulatory updates, key initiatives, and upcoming events.
BRAND NEWS
Pfizer Bets On Medical Cannabis With $6.7 Billion Acquisition
- American multinational pharmaceutical and biotechnology corporation Pfizer signed an agreement with the clinical-stage company Arena Pharmaceuticals for a total equity value of around $6.7 billion, betting on a promising cannabinoid-based bowel disease treatment.
- Arena Pharmaceuticals is a biotech company with one pipeline dedicated to cannabinoid-type therapeutics. The core of its cannabis operation consists of Olorinab (APD371), an investigational, oral, full agonist of the cannabinoid type 2 receptor (CB2), which aims to treat patients with diseases affecting the stomach and intestine. As Arena’s website states, Olorinab is an investigational drug and is not currently approved for use by any health authority.
- Arena’s team is developing this cannabinoid-based drug with an initial focus on visceral pain associated with gastrointestinal disorders. Arena’s website reads that this compound, through its selectivity for CB2 versus CB1, is under investigation for pain relief without psychoactive adverse effects.
Avecho eyes arthritis market with Medterra tie up in the US
- Avecho Biotechnology has entered into a licensing and supply agreement with US CBD company Medterra Pharma to develop and commercialise its soft-gel CBD capsule for the treatment of arthritis.
- Medterra will develop the product with a view to supporting US Food and Drug Administration approval, in parallel with Avecho’s efforts to register it for sleep indications with the TGA in Australia.
- Medterra’s scientists have already demonstrated the therapeutic potential of CBD for arthritis in preclinical models, and a six-week human study using the soft-gel for pain reduction in patients with osteoarthritis of the knee is scheduled for early next year.
- Avecho CEO Dr Paul Gavin said: “While Avecho focuses on a sleep-related indication, this agreement allows Medterra to fund the development of the CBD soft-gel product for an additional indication, providing Avecho with potential upside for an indication we do not have the bandwidth to pursue ourselves.”
CBDMD Reports Fiscal Year Net Sales Increased 6.2% to Record $44.5 Million
- Net sales for CBDMD, Inc., operator of its leading CBD brands – CBDMD, Paw CBD CBDMD Botanicals – increased 6.2% to a record $44.5 million for the fourth quarter and fiscal year ended Sept. 30.
- Nets sales were $41.8 million in fiscal year 2020.
- According to chairman and co-CEO, Martin A. Sumichrast, CBDMD outpaced the industry average despite the market researcher Brightfield Group’s expectation of the US’s CBD market experiencing its most challenging year with year-over-year growth rates anticipated to be a flat 2.5%. Its gross profit margin increased to 67% in fiscal 2021 from 63% in fiscal 2020.
- “In three short years we have grown from an unknown start-up brand competing against thousands of other CBD brands, outpacing the pack to be firmly positioned at the top of the CBD industry, with our three leading brands,” said Sumichrast.
- CBDMD recorded e-commerce, direct to consumer net sales of $32.9 million, or 74.2% of total net sales in fiscal 2021, reflecting an increase of $2.5 million, or 8%, from fiscal 2020. Its CBD pet brand, Paw CBD, reported $5.7 million in net sales, a 27% increase in fiscal 2021, compared to $4.5 million in fiscal 2020.
Executive Shuffle Announced at Charlotte’s Web
- Colorado CBD maker Charlotte’s Web Holdings announced a leadership shakeup, replacing CEO Deanie Elsner with a former Bacardi executive. Jacques Tortoroli was former Chief Administrative Officer of Bacardi Ltd., the world’s largest privately-held spirits company.
- Other executive changes announced by the Boulder, Colorado, company include:
- Chief Financial Officer Wes Booysen getting an expanded role as Chief Financial & Operating Officer.
- Chief Cultivation Officer Jared Stanley taking on an expanded role as Chief Cultivation and Innovation Officer , overseeing extraction and new-product development.The changes come after Charlotte’s Web stock fell some 44% in the last three months of the year.
- Trait Biosciences Inc., a leading cannabinoid biotechnology research organization, announced that it has successfully completed key scientific studies and operational milestones associated with its Series A Financing, and said that Dr. Hanny Kanafani has been appointed to the newly created position of Vice President of Science Technology.
- This announcement follows the company’s recent appointment of business executive Auroni Majumdar of International Flavors and Fragrances to the Trait Advisory Board and the company’s June announcement of a C$31 million Series A financing round led by Btomorrow Ventures Limited (BTV), the corporate venturing unit of British American Tobacco (BAT).
TREND PIECES AND EDITORIAL
Counterculture to Counterintuitive: Cannabis to Help You Diet?
- Sold by Wana Brands, a well-established edibles company, Fit gummies are available only in Wana’s home state of Colorado (for now).
- Wana’s marketing materials state that the product’s weight-management benefits are proven by a 2021 clinical trial that was commissioned by its partner on the gummy, ECS Brands, and supported by the National Institutes of Health. According to the Wana’s website, “the recently completed NIH-supported, 90-day human clinical trial found 100 out of 100 participants in the study lost weight without exercise or changing daily caloric output values.”
- The experts interviewed for this article, including cannabis and pharmacology researchers and clinicians, spotted several red flags in the paper.
- For one, the paper claims that its study was sponsored by the National Institutes of Health, which the N.I.H. denied. The paper makes no mention of ethical oversight and does not appear to have been approved by an institutional review board, a type of group that reviews clinical trials in order to protect human research subjects.
- ECS Brands said in a phone call that the study was approved by a review board but would not provide additional details or confirm such information in writing.
- Experts also pointed out that the paper does not state where the trial was conducted or how subjects were recruited. ECS Brands’ informational sheet on the trial says it was performed under the guidance of the Mayo Clinic. Both the N.I.H. and the Mayo Clinic said they had no record of the trial, nor is it registered on ClinicalTrials.gov. The results of the trial have not been published in a scientific journal or peer-reviewed.
- And, they said, the numbers seem too good to be true. In marketing materials, ECS Brands says that 100 out of 100 participants who took its product lost weight. “I’ve seen very few studies where anything works 100 percent of the time,” Dr. Englund said.
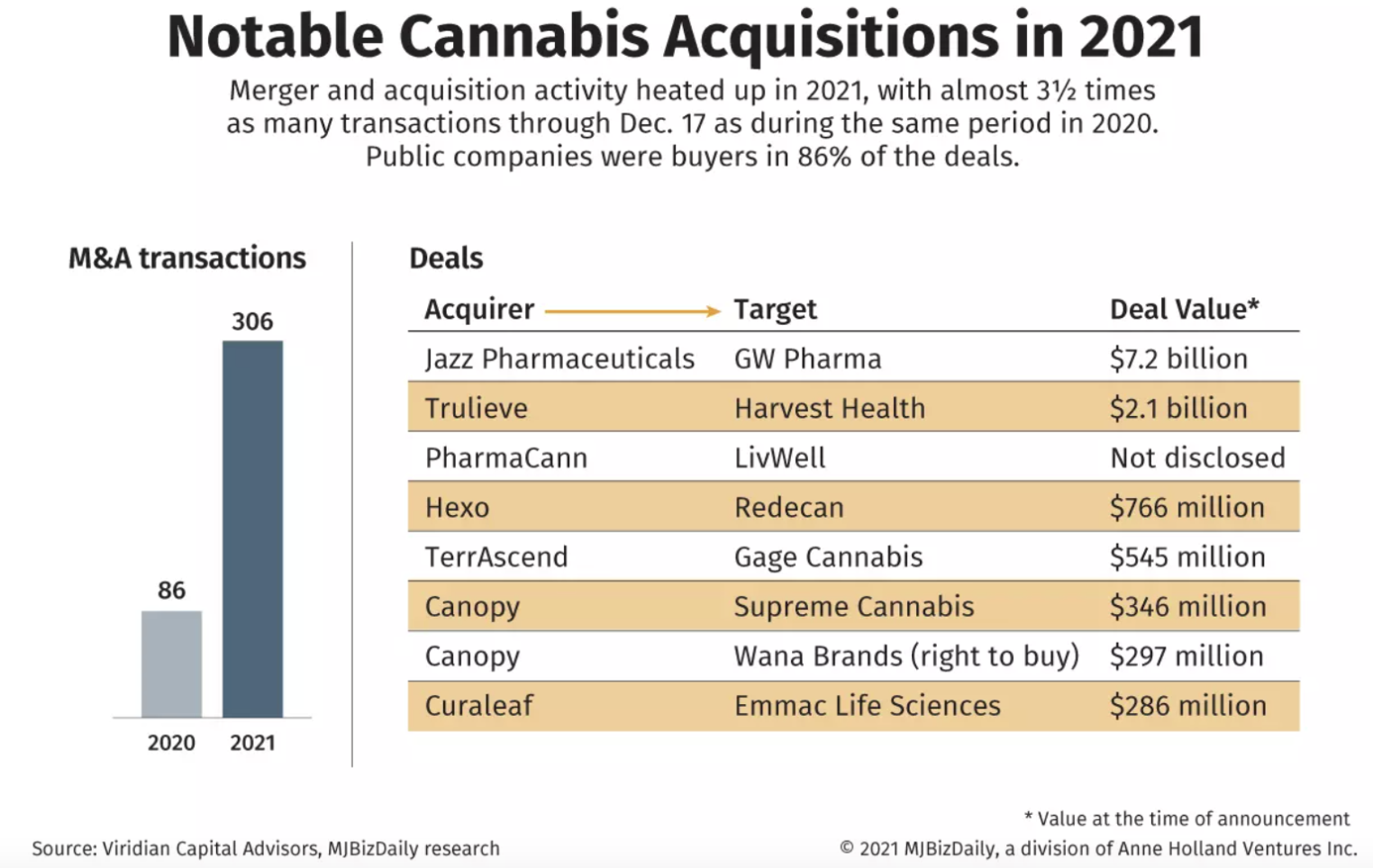
- Marijuana merger and acquisition activity proceeded at a torrid pace in 2021 – and could accelerate in 2022 – thanks to lower interest costs and pressure on larger companies to expand their footprints and boost revenue.
- New York-based Viridian Capital Advisors counted 306 M&A transactions through Dec. 17, up well over threefold from the 86 recorded for the same period of 2020, including 209 in the U.S. totaling $10.1 billion in value – with both the numbers and amount exceeding what was recorded in 2019 and 2020 combined.
The year included a couple of blockbuster, multibillion-dollar deals:
- Dublin-based global pharma company Jazz Pharmaceuticals agreed to buy United Kingdom-based GW Pharmaceuticals, one of the largest medical cannabinoid companies in the world, for $7.2 billion. The transaction diversifies Jazz’s commercial portfolio pipeline with therapies that are complementary to its existing business. GW is a proven cannabis biotech firm and has successfully commercialized US Food and Drug Administration (FDA) approved cannabinoid-based prescription medicines. The acquisition (the largest in the history of the sector) was a landmark transaction for the cannabis industry and increases the credibility that is associated with it.
- American multinational pharmaceutical and biotechnology corporation Pfizer signed an agreement with the clinical-stage company Arena Pharmaceuticals for a total equity value of around $6.7 billion.
- Florida-based multistate marijuana operator Trulieve Cannabis acquired Arizona-based Harvest Health & Recreation in a deal initially valued at $2.1 billion.
- This year’s figures don’t include the multibillion-dollar merger of the Canadian cannabis producers Tilray and Aphria, which was announced in late 2020 but completed in 2021.
How the Cannabis Industry Is Embracing the CPG Model
- If the industry is making progress in its 2.0 stage, what will 3.0 look like?
- The breadth of offerings that seems to characterize Cannabis 2.0 may become narrower in the next stage of growth. Companies will get more focused and really try to win and own a more limited SKU set and a more limited product offering within a category versus the kind of breadth today that exists with a lot of the leading operators.
- What those more limited SKU sets look like for companies could evolve beyond what is available in the market today. “Today, higher dose products are still king. When cannabis becomes federally legal, you’ll also see micro-dosed products rapidly increase in popularity as new-to-cannabis consumers flood into the market.”
- The retail stores, too, are likely to change as the industry matures. As more and more mainstream cannabis products enter the market, the cannabis industry’s retail environments will look and feel a lot more like grocery and liquor stores. Cannabis 3.0 is the convergence of cannabis and the traditional CPG markets into one: for example, buying Cann at a liquor store or a bar, having beer distributors deliver cannabis products and alcohol products using the same trucks, manufacturing lines that run both alcoholic beverages and cannabis beverages.
Big Opportunities in Merging Nutra and Pharma
- Consumer trends, technological advancements and business strategies are causing the pharma and nutra markets to merge and this opens opportunities for the players in both industries to cater to consumers looking for the ultimate wellness solutions.
- “We don’t have separate camps of dietary supplement consumers and OTC consumers. We have consumers who are trying to feel better and maintain a better quality of life, and those consumers stand to benefit from both markets. There was a long time in the dietary supplement world where companies were offering alternative solutions to drugs and I don’t think this is our best space – I think our mandate is supporting wellness.”
- GOOD: The merging of the markets can lead to products that are actually better for you, made to the highest global standards in terms of purity, potency, safety, sustainability, equity, and social justice.
- For nutra, if we get this right, it drives trust, For pharma, it will give them a diversity of offerings and new opportunities to participate in making lives better.
- CLEAN: Consumers are demanding clean label food and drugs, with a revolution already happening in the OTC category.
- FUN: Consumers are getting bored with the same old products, leading to the rising interest in gummy supplements as well as other formats including strips, shots, melts, lozenges, pastilles, and solid emulsions.
-
The mantra for formulating products this year is ABC – anything but capsules.
REGULATORY
Increased THC limit in Europe is a positive next step for trade, hemp insiders say
- Hemp entrepreneurs worldwide cheered last month when European lawmakers finalized work raising the THC limit for hemp crops from 0.2% to 0.3% total THC. The new policy, which further aligns the EU’s THC limit with the legal THC threshold for hemp in Canada and the United States, takes effect in 2023.
- According to the European Industrial Hemp Association, the change allows farmers to receive direct payments for registered hemp varieties that have a maximum level of 0.3% THC – providing more choices in genetics for producers.
- In some countries in Europe, such as Italy and the Czech Republic, farmers are permitted to grow hemp with THC on the field over 0.3%, but those farmers would not be eligible for direct payments under EU rules.
- Increasing the THC limit is a positive small step forward indicating that EU lawmakers “are closer to fully acknowledging and recognizing the existence of a legitimate European hemp sector,” EIHA Managing Director Lorenza Romanese said in a statement.
ACMD advice on consumer cannabidiol (CBD) products (UK)
- The ministerial commission asked the ACMD to specify which controlled cannabinoids should be controlled and set an unavoidable trace level for each of these within consumer CBD products. The following conclusions were reached:
- The total dose of ∆9-THC (including ∆9-THCA, as calculated using Equation 1 in the report) and all other controlled phytocannabinoids in consumer CBD products be controlled. The dose of each controlled phytocannabinoid should not exceed 50 micrograms (µg) per unit of consumption.
- Regulatory authorities ensure that any consumer CBD product permitted to market has limits on the content of controlled phytocannabinoids such that the dose of ∆9-THC (including its precursor ∆9-THCA) and of each of the other controlled phytocannabinoids does not exceed 50 micrograms (µg) per unit of consumption
- A further inter laboratory comparison trial (ring trial) should be commissioned specifically to support the capability of testing laboratories to detect controlled phytocannabinoids below the recommended maximum levels in a representative range of consumer CBD products
NY loosens rules on CBD product potency, shelf stability and THC warnings
- New York regulators have loosened CBD restrictions including potency limits, serving sizes and warnings about THC levels.
- The new rules approved Thursday by New York’s Cannabis Control Board, which oversees both the hemp and marijuana industries, change the per-serving CBD limit in dietary supplements from 75 milligrams to 100 milligrams.
- Regulators also removed a requirement that cannabinoid hemp products be shelf stable, a move to allow foods and drinks infused with cannabinoids.
CATEGORY & RESEARCH TRENDS
FDA Signs Off on Human CBD Trials to Treat Opioid Addiction
- U.S. health authorities are signing off on human trials of CBD to treat opioid addiction.
- The U.S. Food and Drug Administration gave the green light this week to Ananda Scientific to start human testing of its CBD drug Nantheia ATL5, the company announced.
- The trials will be run out of UCLA and are funded by the U.S. National Institute on Drug Abuse.
Turmeric extracts may help with weight loss, inflammatory profiles, & mental health: Study
- Twelve weeks of supplementation with a turmeric extract led to significant reductions in body weight for overweight but healthy people. The extract was also associated with improvements in mental health scores.
Glanbia Nutritionals to use microencapsulation to boost ashwagandha efficacy
- Glanbia Nutritionals has formalised a partnership with Ixoreal Biomed to optimize high concentration KSM-66 ashwagandha root extract and enhance functionality.
Aurora and 22nd Century Group License Foundational Biosynthesis IP to Cronos Group
- Aurora Cannabis Inc., the Canadian company defining the future of cannabinoids worldwide, together with 22nd Century Group, Inc. announced today a three-way non-exclusive agreement to license biosynthesis intellectual property to Cronos Group Inc., intended to assist in the advancement of research and development on the biosynthesis of cannabinoids.
- Biosynthesis, a process common in the pharmaceutical industry, involves using living microorganisms to convert simple substances into complex compounds. Through biosynthesis, cannabinoids, particularly those that are rare such as cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), are expected to be produced efficiently and reliably at high levels of purity.
- Aurora’s connection to the biosynthetic production of cannabinoids originated with early work carried out by the Company’s former Chief Science Officer on the discovery of key genes within the cannabinoid biosynthesis pathway.
The Valens Company Announces Two Strategic Agreements with PMI Mexico
- Under the first agreement, Valens will supply CBD for PMI’s ongoing pharmacokinetic (PK) stage medical trials, which are focused on anti-inflammatory applications of medical-grade, nano-water emulsified CBD oil. The clinical trial has already been approved by The Medical Ethics Board of Mexico. The clinical trial, which is set to begin once Valens shipments arrive in Q1 of 2022, will be conducted across private hospitals in Mexico. At the conclusion of the trial, Valens will be the sole manufacturer and global distributor in collaboration with PMI.
- Under the second agreement, Valens will manufacture and distribute CBD-infused and uninfused PredilifeⓇ products globally while PMI will be responsible for distribution of the PredilifeⓇ products in Mexico. Predilife is an agave-based prebiotic powder that has been submitted as a drug master file” (DMF) to the FDA. Valens will manufacture the products at its Green Roads facility in Florida, where it will be infused with CBD as required and then packaged and distributed through Valens’ third-party supplier channels across Asia, U.S., Latin America and Mexico. The formulation will be offered in both powder and liquid sachets, initially for human consumption, with animal variations planned for the second half of 2022.
UPCOMING EVENTS & WEBINARS
- Champs Vegas, (February 2 – 5, Las Vegas, NV)
- Emerald Conference, (February 27 – March 1, San Diego, CA)
- Arnold Classic, (March 3 – 6, Columbus, OH)
- Expo West, (March 8 – 12, Anaheim, CA)
Got Innovation? If you see an interesting new brand, trend or other innovative idea, please feel free to share directly to nicole@openbookextracts. All ideas are welcome!
Author: Nicole Brown
January 10 – 10 min read
What’s new, what’s next, and what it means for our business.
Published monthly, the OBX Innovation Briefing is presented by the OBX team as a way of highlighting the latest developments and innovations within the global cannabinoid industry. Each curated volume features insights and commentary on industry and category trends, regulatory updates, key initiatives, and upcoming events.
BRAND NEWS
Pfizer Bets On Medical Cannabis With $6.7 Billion Acquisition
- American multinational pharmaceutical and biotechnology corporation Pfizer signed an agreement with the clinical-stage company Arena Pharmaceuticals for a total equity value of around $6.7 billion, betting on a promising cannabinoid-based bowel disease treatment.
- Arena Pharmaceuticals is a biotech company with one pipeline dedicated to cannabinoid-type therapeutics. The core of its cannabis operation consists of Olorinab (APD371), an investigational, oral, full agonist of the cannabinoid type 2 receptor (CB2), which aims to treat patients with diseases affecting the stomach and intestine. As Arena’s website states, Olorinab is an investigational drug and is not currently approved for use by any health authority.
- Arena’s team is developing this cannabinoid-based drug with an initial focus on visceral pain associated with gastrointestinal disorders. Arena’s website reads that this compound, through its selectivity for CB2 versus CB1, is under investigation for pain relief without psychoactive adverse effects.
Avecho eyes arthritis market with Medterra tie up in the US
- Avecho Biotechnology has entered into a licensing and supply agreement with US CBD company Medterra Pharma to develop and commercialise its soft-gel CBD capsule for the treatment of arthritis.
- Medterra will develop the product with a view to supporting US Food and Drug Administration approval, in parallel with Avecho’s efforts to register it for sleep indications with the TGA in Australia.
- Medterra’s scientists have already demonstrated the therapeutic potential of CBD for arthritis in preclinical models, and a six-week human study using the soft-gel for pain reduction in patients with osteoarthritis of the knee is scheduled for early next year.
- Avecho CEO Dr Paul Gavin said: “While Avecho focuses on a sleep-related indication, this agreement allows Medterra to fund the development of the CBD soft-gel product for an additional indication, providing Avecho with potential upside for an indication we do not have the bandwidth to pursue ourselves.”
CBDMD Reports Fiscal Year Net Sales Increased 6.2% to Record $44.5 Million
- Net sales for CBDMD, Inc., operator of its leading CBD brands – CBDMD, Paw CBD CBDMD Botanicals – increased 6.2% to a record $44.5 million for the fourth quarter and fiscal year ended Sept. 30.
- Nets sales were $41.8 million in fiscal year 2020.
- According to chairman and co-CEO, Martin A. Sumichrast, CBDMD outpaced the industry average despite the market researcher Brightfield Group’s expectation of the US’s CBD market experiencing its most challenging year with year-over-year growth rates anticipated to be a flat 2.5%. Its gross profit margin increased to 67% in fiscal 2021 from 63% in fiscal 2020.
- “In three short years we have grown from an unknown start-up brand competing against thousands of other CBD brands, outpacing the pack to be firmly positioned at the top of the CBD industry, with our three leading brands,” said Sumichrast.
- CBDMD recorded e-commerce, direct to consumer net sales of $32.9 million, or 74.2% of total net sales in fiscal 2021, reflecting an increase of $2.5 million, or 8%, from fiscal 2020. Its CBD pet brand, Paw CBD, reported $5.7 million in net sales, a 27% increase in fiscal 2021, compared to $4.5 million in fiscal 2020.
Executive Shuffle Announced at Charlotte’s Web
- Colorado CBD maker Charlotte’s Web Holdings announced a leadership shakeup, replacing CEO Deanie Elsner with a former Bacardi executive. Jacques Tortoroli was former Chief Administrative Officer of Bacardi Ltd., the world’s largest privately-held spirits company.
- Other executive changes announced by the Boulder, Colorado, company include:
- Chief Financial Officer Wes Booysen getting an expanded role as Chief Financial & Operating Officer.
- Chief Cultivation Officer Jared Stanley taking on an expanded role as Chief Cultivation and Innovation Officer , overseeing extraction and new-product development.
- The changes come after Charlotte’s Web stock fell some 44% in the last three months of the year.
- Trait Biosciences Inc., a leading cannabinoid biotechnology research organization, announced that it has successfully completed key scientific studies and operational milestones associated with its Series A Financing, and said that Dr. Hanny Kanafani has been appointed to the newly created position of Vice President of Science Technology.
- This announcement follows the company’s recent appointment of business executive Auroni Majumdar of International Flavors and Fragrances to the Trait Advisory Board and the company’s June announcement of a C$31 million Series A financing round led by Btomorrow Ventures Limited (BTV), the corporate venturing unit of British American Tobacco (BAT).
TREND PIECES AND EDITORIAL
Counterculture to Counterintuitive: Cannabis to Help You Diet?
- Sold by Wana Brands, a well-established edibles company, Fit gummies are available only in Wana’s home state of Colorado (for now).
- Wana’s marketing materials state that the product’s weight-management benefits are proven by a 2021 clinical trial that was commissioned by its partner on the gummy, ECS Brands, and supported by the National Institutes of Health. According to the Wana’s website, “the recently completed NIH-supported, 90-day human clinical trial found 100 out of 100 participants in the study lost weight without exercise or changing daily caloric output values.”
- The experts interviewed for this article, including cannabis and pharmacology researchers and clinicians, spotted several red flags in the paper.
- For one, the paper claims that its study was sponsored by the National Institutes of Health, which the N.I.H. denied. The paper makes no mention of ethical oversight and does not appear to have been approved by an institutional review board, a type of group that reviews clinical trials in order to protect human research subjects.
- ECS Brands said in a phone call that the study was approved by a review board but would not provide additional details or confirm such information in writing.
- Experts also pointed out that the paper does not state where the trial was conducted or how subjects were recruited. ECS Brands’ informational sheet on the trial says it was performed under the guidance of the Mayo Clinic. Both the N.I.H. and the Mayo Clinic said they had no record of the trial, nor is it registered on ClinicalTrials.gov. The results of the trial have not been published in a scientific journal or peer-reviewed.
- And, they said, the numbers seem too good to be true. In marketing materials, ECS Brands says that 100 out of 100 participants who took its product lost weight. “I’ve seen very few studies where anything works 100 percent of the time,” Dr. Englund said.
- Marijuana merger and acquisition activity proceeded at a torrid pace in 2021 – and could accelerate in 2022 – thanks to lower interest costs and pressure on larger companies to expand their footprints and boost revenue.
- New York-based Viridian Capital Advisors counted 306 M&A transactions through Dec. 17, up well over threefold from the 86 recorded for the same period of 2020, including 209 in the U.S. totaling $10.1 billion in value – with both the numbers and amount exceeding what was recorded in 2019 and 2020 combined.
The year included a couple of blockbuster, multibillion-dollar deals:
- Dublin-based global pharma company Jazz Pharmaceuticals agreed to buy United Kingdom-based GW Pharmaceuticals, one of the largest medical cannabinoid companies in the world, for $7.2 billion. The transaction diversifies Jazz’s commercial portfolio pipeline with therapies that are complementary to its existing business. GW is a proven cannabis biotech firm and has successfully commercialized US Food and Drug Administration (FDA) approved cannabinoid-based prescription medicines. The acquisition (the largest in the history of the sector) was a landmark transaction for the cannabis industry and increases the credibility that is associated with it.
- American multinational pharmaceutical and biotechnology corporation Pfizer signed an agreement with the clinical-stage company Arena Pharmaceuticals for a total equity value of around $6.7 billion.
- Florida-based multistate marijuana operator Trulieve Cannabis acquired Arizona-based Harvest Health & Recreation in a deal initially valued at $2.1 billion.
- This year’s figures don’t include the multibillion-dollar merger of the Canadian cannabis producers Tilray and Aphria, which was announced in late 2020 but completed in 2021.
How the Cannabis Industry Is Embracing the CPG Model
- If the industry is making progress in its 2.0 stage, what will 3.0 look like?
- The breadth of offerings that seems to characterize Cannabis 2.0 may become narrower in the next stage of growth. Companies will get more focused and really try to win and own a more limited SKU set and a more limited product offering within a category versus the kind of breadth today that exists with a lot of the leading operators.
- What those more limited SKU sets look like for companies could evolve beyond what is available in the market today. “Today, higher dose products are still king. When cannabis becomes federally legal, you’ll also see micro-dosed products rapidly increase in popularity as new-to-cannabis consumers flood into the market.”
- The retail stores, too, are likely to change as the industry matures. As more and more mainstream cannabis products enter the market, the cannabis industry’s retail environments will look and feel a lot more like grocery and liquor stores. Cannabis 3.0 is the convergence of cannabis and the traditional CPG markets into one: for example, buying Cann at a liquor store or a bar, having beer distributors deliver cannabis products and alcohol products using the same trucks, manufacturing lines that run both alcoholic beverages and cannabis beverages.
Big Opportunities in Merging Nutra and Pharma
- Consumer trends, technological advancements and business strategies are causing the pharma and nutra markets to merge and this opens opportunities for the players in both industries to cater to consumers looking for the ultimate wellness solutions.
- “We don’t have separate camps of dietary supplement consumers and OTC consumers. We have consumers who are trying to feel better and maintain a better quality of life, and those consumers stand to benefit from both markets. There was a long time in the dietary supplement world where companies were offering alternative solutions to drugs and I don’t think this is our best space – I think our mandate is supporting wellness.”
- GOOD: The merging of the markets can lead to products that are actually better for you, made to the highest global standards in terms of purity, potency, safety, sustainability, equity, and social justice.
- For nutra, if we get this right, it drives trust, For pharma, it will give them a diversity of offerings and new opportunities to participate in making lives better.
- CLEAN: Consumers are demanding clean label food and drugs, with a revolution already happening in the OTC category.
- FUN: Consumers are getting bored with the same old products, leading to the rising interest in gummy supplements as well as other formats including strips, shots, melts, lozenges, pastilles, and solid emulsions.
-
The mantra for formulating products this year is ABC – anything but capsules.
REGULATORY
Increased THC limit in Europe is a positive next step for trade, hemp insiders say
- Hemp entrepreneurs worldwide cheered last month when European lawmakers finalized work raising the THC limit for hemp crops from 0.2% to 0.3% total THC. The new policy, which further aligns the EU’s THC limit with the legal THC threshold for hemp in Canada and the United States, takes effect in 2023.
- According to the European Industrial Hemp Association, the change allows farmers to receive direct payments for registered hemp varieties that have a maximum level of 0.3% THC – providing more choices in genetics for producers.
- In some countries in Europe, such as Italy and the Czech Republic, farmers are permitted to grow hemp with THC on the field over 0.3%, but those farmers would not be eligible for direct payments under EU rules.
- Increasing the THC limit is a positive small step forward indicating that EU lawmakers “are closer to fully acknowledging and recognizing the existence of a legitimate European hemp sector,” EIHA Managing Director Lorenza Romanese said in a statement.
ACMD advice on consumer cannabidiol (CBD) products (UK)
- The ministerial commission asked the ACMD to specify which controlled cannabinoids should be controlled and set an unavoidable trace level for each of these within consumer CBD products. The following conclusions were reached:
- The total dose of ∆9-THC (including ∆9-THCA, as calculated using Equation 1 in the report) and all other controlled phytocannabinoids in consumer CBD products be controlled. The dose of each controlled phytocannabinoid should not exceed 50 micrograms (µg) per unit of consumption.
- Regulatory authorities ensure that any consumer CBD product permitted to market has limits on the content of controlled phytocannabinoids such that the dose of ∆9-THC (including its precursor ∆9-THCA) and of each of the other controlled phytocannabinoids does not exceed 50 micrograms (µg) per unit of consumption
- A further inter laboratory comparison trial (ring trial) should be commissioned specifically to support the capability of testing laboratories to detect controlled phytocannabinoids below the recommended maximum levels in a representative range of consumer CBD products
NY loosens rules on CBD product potency, shelf stability and THC warnings
- New York regulators have loosened CBD restrictions including potency limits, serving sizes and warnings about THC levels.
- The new rules approved Thursday by New York’s Cannabis Control Board, which oversees both the hemp and marijuana industries, change the per-serving CBD limit in dietary supplements from 75 milligrams to 100 milligrams.
- Regulators also removed a requirement that cannabinoid hemp products be shelf stable, a move to allow foods and drinks infused with cannabinoids.
CATEGORY & RESEARCH TRENDS
FDA Signs Off on Human CBD Trials to Treat Opioid Addiction
- U.S. health authorities are signing off on human trials of CBD to treat opioid addiction.
- The U.S. Food and Drug Administration gave the green light this week to Ananda Scientific to start human testing of its CBD drug Nantheia ATL5, the company announced.
- The trials will be run out of UCLA and are funded by the U.S. National Institute on Drug Abuse.
Turmeric extracts may help with weight loss, inflammatory profiles, & mental health: Study
- Twelve weeks of supplementation with a turmeric extract led to significant reductions in body weight for overweight but healthy people. The extract was also associated with improvements in mental health scores.
Glanbia Nutritionals to use microencapsulation to boost ashwagandha efficacy
- Glanbia Nutritionals has formalised a partnership with Ixoreal Biomed to optimize high concentration KSM-66 ashwagandha root extract and enhance functionality.
Aurora and 22nd Century Group License Foundational Biosynthesis IP to Cronos Group
- Aurora Cannabis Inc., the Canadian company defining the future of cannabinoids worldwide, together with 22nd Century Group, Inc. announced today a three-way non-exclusive agreement to license biosynthesis intellectual property to Cronos Group Inc., intended to assist in the advancement of research and development on the biosynthesis of cannabinoids.
- Biosynthesis, a process common in the pharmaceutical industry, involves using living microorganisms to convert simple substances into complex compounds. Through biosynthesis, cannabinoids, particularly those that are rare such as cannabigerol (CBG), cannabichromene (CBC) and cannabinol (CBN), are expected to be produced efficiently and reliably at high levels of purity.
- Aurora’s connection to the biosynthetic production of cannabinoids originated with early work carried out by the Company’s former Chief Science Officer on the discovery of key genes within the cannabinoid biosynthesis pathway.
The Valens Company Announces Two Strategic Agreements with PMI Mexico
- Under the first agreement, Valens will supply CBD for PMI’s ongoing pharmacokinetic (PK) stage medical trials, which are focused on anti-inflammatory applications of medical-grade, nano-water emulsified CBD oil. The clinical trial has already been approved by The Medical Ethics Board of Mexico. The clinical trial, which is set to begin once Valens shipments arrive in Q1 of 2022, will be conducted across private hospitals in Mexico. At the conclusion of the trial, Valens will be the sole manufacturer and global distributor in collaboration with PMI.
- Under the second agreement, Valens will manufacture and distribute CBD-infused and uninfused PredilifeⓇ products globally while PMI will be responsible for distribution of the PredilifeⓇ products in Mexico. Predilife is an agave-based prebiotic powder that has been submitted as a drug master file” (DMF) to the FDA. Valens will manufacture the products at its Green Roads facility in Florida, where it will be infused with CBD as required and then packaged and distributed through Valens’ third-party supplier channels across Asia, U.S., Latin America and Mexico. The formulation will be offered in both powder and liquid sachets, initially for human consumption, with animal variations planned for the second half of 2022.
UPCOMING EVENTS & WEBINARS
- Champs Vegas, (February 2 – 5, Las Vegas, NV)
- Emerald Conference, (February 27 – March 1, San Diego, CA)
- Arnold Classic, (March 3 – 6, Columbus, OH)
- Expo West, (March 8 – 12, Anaheim, CA)
Got Innovation? If you see an interesting new brand, trend or other innovative idea, please feel free to share directly to nicole@openbookextracts. All ideas are welcome!